
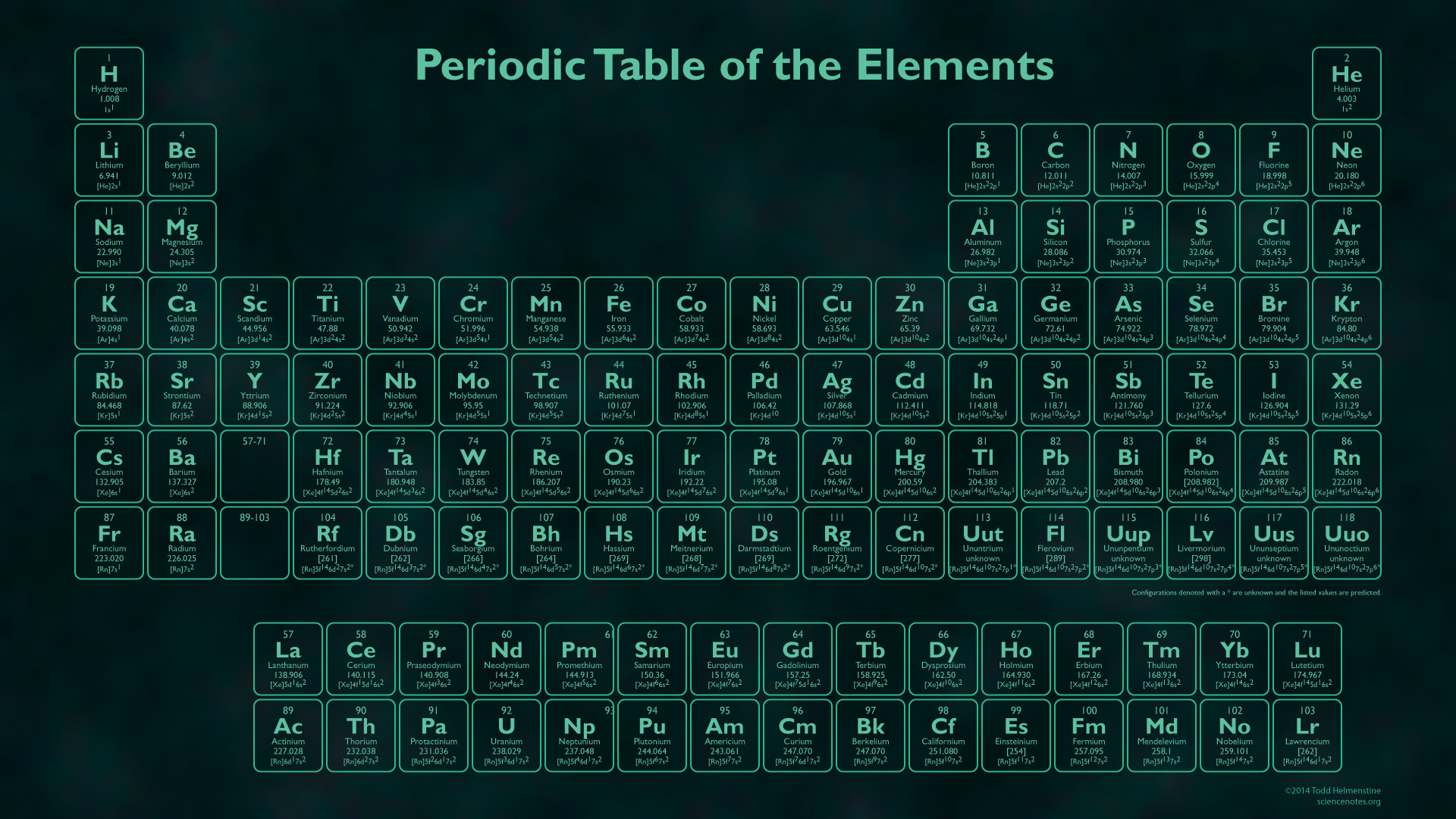
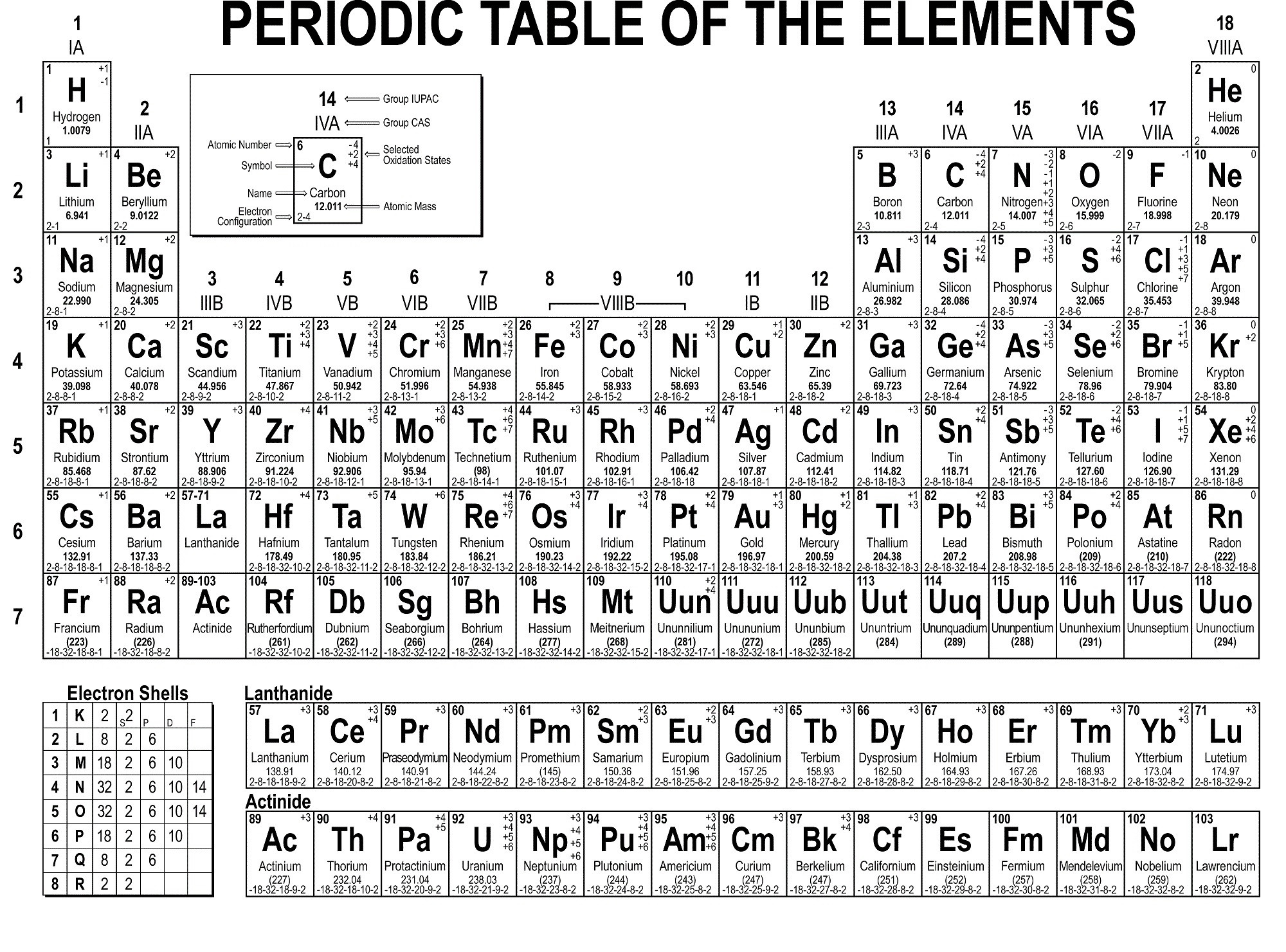
In simple terms, the first element in each row starts a new shell containing one electron, while the last element in each row has two (or one for the the first row) of the subshells in the outer shell fully occupied. Each shell has subshells, and the order in which the shells/subshells get filled is based on the energy required, although it’s a complicated process.
#Complete periodic table chemistry series#
Think of the atom with a central nucleus that contains all the protons and neutrons, surrounded by a series of shells that contain the electrons.Įach row in the periodic table sort of corresponds to filling up one of these shells with electrons. We tend to think of atoms as built a bit like onions, with seven layers of electrons called “shells”, labelled K, L, M, N, O, P, and Q, surrounding the core nucleus.

This isotope is a radioactive version of normal carbon C (or carbon-12) that has two extra neutrons.īut why is there a separate box of elements below the main table, and why is the main table an odd shape, with a bite taken out of the top? That comes down to how the other component of the atom – the electrons – are arranged. This gives rise to different isotopes for every element.įor example, you may have heard of carbon-14 dating to determine the age of objects. The number of neutrons in the nucleus can vary. It ranges from element 1 (hydrogen H) in the top left, to the newly approved element 118 (oganesson Og) in the bottom right. The periodic table is arranged in order of increasing atomic number (left to right, top to bottom). The properties of hydrogen as marked on the periodic table. It is the number of protons that gives an element its atomic number – the number generally found in the top left corner of each box in the periodic table. Atoms have a central core (the nucleus) made up of smaller particles called protons and neutrons. To really understand the final structure of the periodic table, we need to understand a bit about atoms and how they are constructed. Over time, these gaps were filled in and the final version as we know it today emerged. Importantly, Mendeleev left gaps in the table where he thought missing elements should be placed. Mendeleev’s periodic table is first published outside Russia in Zeitschrift für Chemie (1869, pages 405-6). The first version of the current table is generally attributed to Russian chemistry professor Dmitri Mendeleev in 1869, with an updated version in 1871. This left gaps, which made deciphering patterns a bit like trying to assemble a jigsaw puzzle with missing pieces.ĭifferent scientists came up with different types of tables. The task was made more difficult because not all of the elements were known. Scientists began to look at ways to arrange them systematically so that similar properties could be grouped together, just as someone collecting seashells might try to organise them by shape or colour. At the time when elements were first being discovered, the structure of atoms was not known. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License. We recommend using aĪuthors: Paul Flowers, Klaus Theopold, Richard Langley, William R. Use the information below to generate a citation. Then you must include on every digital page view the following attribution: If you are redistributing all or part of this book in a digital format, Then you must include on every physical page the following attribution:

If you are redistributing all or part of this book in a print format, Want to cite, share, or modify this book? This book uses the


 0 kommentar(er)
0 kommentar(er)
